價格:免費
更新日期:2018-08-30
檔案大小:26.7 MB
目前版本:2.6
版本需求:需要 iOS 9.0 或以上版本。與 iPhone、iPad 及 iPod touch 相容。
支援語言:英語

The NbN was developed because it has become clear that the current pharmacological nomenclature of psychotropic medications does not reflect our contemporary knowledge, nor does it appropriately inform the clinician about neuroscience-based prescribing. Very often we prescribe “antidepressants” for “anxiety” disorders or “second generation antipsychotics” to depressed patients.
This practice is confusing.
• The mission of NbN is to embed our current neuroscience advances in the nomenclature.
• The scope is to include all medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
This proposed nomenclature aims to reflect the current pharmacology knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled this app could have taken the stand that our current knowledge base is not enough to define the exact pharmacological domain or the correct mechanisms of action. However, as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. After all, we need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:-
1. The need to treat now
2. Updated neuroscience insights
3. The judgment of the members of the taskforce
4. The assumption that children are developing and therefore developmental aspects (e.g. liver and kidney function, brain developmental stage) must be considered in use and dosage.
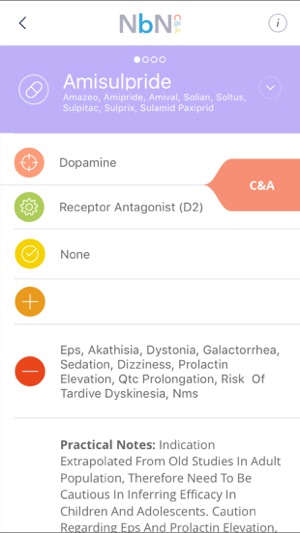
Along these lines, we have come up with a nomenclature that is driven by: pharmacology and mode of action. The NbN reflects current knowledge and understanding regarding neurotransmitters / molecules / systems being modified + mode / mechanism of action
4 clinically relevant dimensions are also included: 1.Approved indications – Based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.) for this age group.
2. Efficacy and side effects – Aimed to highlight situations in which a compound has no formal approval for an indication, yet there is evidence to support its use in additional indication(s), for example well supported expert guidelines.
In the side effects section, only serious, life-changing or prevalent side-effects are listed. 3. “Practical notes” – Summarizes the clinical knowledge that has been "filtered" though the taskforce "sieve".
4. Neurobiology – Derived from empirical data.
For those who would like to know more about the pharmacology there is a direct link to the relevant site of IUPHAR – our collaborator in this endeavor.
As this is an on-going process, we recognize that there are going to be omissions. Based on your feedback, other colleagues’ responses (the “wisdom of clinicians”), new reports and new findings, appropriate updates will be undertaken.
NbN C&A embeds current neuroscience advances in order to provide a comprehensive and coherent naming system for children and adolescence. The taskforce hopes that it will help clinicians to make informed decisions in their "psychopharmacological steps" and that it clarifies the rationale for prescribing.

支援平台:iPhone
